Hyperthermophilic Enzymes: Sources, Uses, and Molecular Mechanisms for Thermostability
Several properties of
Arg residues suggest that they would be better
adapted to high temperatures than
Lys residues: the Arg
δ-guanido moiety
has a reduced chemical reactivity due to its high pK
a and
its resonance stabilization. The
δ-guanido moiety provides more surface
area for charged interactions than the Lys amino group does.
Figure4 illustrates the ability of Arg to participate in multiple noncovalent
interactions. Because the Arg side chain contains one fewer
methylene
group than Lys, it has the potential to develop less
unfavorable
contacts with the solvent.
Last, because its pK
a
(approximately 12) is 1 unit above that of Lys (11.1), Arg more easily
maintains ion pairs and a net positive charge at elevated temperatures
(pK
a values drop as the temperature increases) (
252,
354).
The average Arg/Lys ratios in the protein pools of the mesophiles and hyperthermophiles listed in Table
(0.73 ± 0.37 and 0.87 ± 0.60, respectively) are associated with large
standard deviations. (Among hyperthermophiles, Arg/Lys ratios vary from
0.52 in
Aquifex aeolicus proteins to 2.19 in
Aeropyrum pernix
proteins.) These results suggest that if an increased Arg content is
indeed stabilizing, this mechanism is not universally used among
hyperthermophiles.
Stereo view of the ion pair between Arg19 and Asp111 in
S. solfataricus
indole-3-glycerol phosphate synthase. The Arg19 guanidinium group also
forms a cation-π interaction with the Tyr93 π system and two H bonds
with Thr84. Reprinted from reference
185 with permission of the publisher.
Arginine residues as stabilizing elements in proteins
Site-specific substitutions of arginine for lysine in the thermostable
D-xylose isomerase (XI) from Actinoplanes missouriensis are shown to
impart significant heat stability enhancement in the presence of sugar
substrates most probably by interfering with nonenzymatic glycation. The
same substitutions are also found to increase heat stability in the
absence of any sugar derivatives, where a mechanism based on prevention
of glycation can no longer be invoked. This rather conservative
substitution is moreover shown to improve thermostability in two other
structurally unrelated proteins, human copper, zinc-superoxide dismutase
(CuZnSOD) and D-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) from
Bacillus subtilis. The stabilizing effect of Lys----Arg substitutions is
rationalized on the basis of a detailed analysis of the crystal
structures of wild-type XI and of engineered variants with Lys----Arg
substitution at four distinct locations, residues 253, 309, 319, and
323. Molecular model building analysis of the structures of wild-type
and mutant CuZnSOD (K9R) and GAPDH (G281K and G281R) is used to explain
the observed stability enhancement in these proteins. In addition to
demonstrating that even thermostable proteins can lend themselves to
further stability improvement, our findings provide direct evidence that
arginine residues are important stabilizing elements in proteins.
Moreover, the stabilizing role of electrostatic interactions,
particularly between subunits in oligomeric proteins, is documented.
A structural role for arginine in proteins: multiple hydrogen bonds to backbone carbonyl oxygens.
We propose that arginine side chains often play a previously
unappreciated general structural role in the maintenance of tertiary
structure in proteins, wherein the positively charged guanidinium group
forms multiple hydrogen bonds to backbone carbonyl oxygens. Using as a
criterion for a "structural" arginine one that forms 4 or more hydrogen
bonds to 3 or more backbone carbonyl oxygens, we have used molecular
graphics to locate arginines of interest in 4 proteins: Arg 180 in
Thermus thermophilus manganese superoxide dismutase, Arg 254 in human
carbonic anhydrase II, Arg 31 in Streptomyces rubiginosus xylose
isomerase, and Arg 313 in Rhodospirillum rubrum
ribulose-1,5-bisphosphate carboxylase/oxygenase. Arg 180 helps to mold
the active site channel of superoxide dismutase, whereas in each of the
other enzymes the structural arginine is buried in the "mantle" (i.e.,
inside, but near the surface) of the protein interior well removed from
the active site, where it makes 5 hydrogen bonds to 4 backbone carbonyl
oxygens. Using a more relaxed criterion of 3 or more hydrogen bonds to 2
or more backbone carbonyl oxygens, arginines that play a potentially
important structural role were found in yeast enolase, Bacillus
stearothermophilus glyceraldehyde-3-phosphate dehydrogenase,
bacteriophage T4 and human lysozymes, Enteromorpha prolifera
plastocyanin, HIV-1 protease, Trypanosoma brucei brucei and yeast
triosephosphate isomerases, and Escherichia coli trp aporepressor (but
not trp repressor or the trp repressor/operator complex)
ps.
moiety 一半, (两个组成部分中的一)部分
Link1
5. Draw the predominant form of arginine at pH 7.4 and 12. Important pK's for the
functional groups in this amino acid are
α-carboxyl=2,
δ-guanido=12,
α-amino=9. What is the ratio of conjugate base/acid for the
δ-guanido group in arginine at pH 7.4 and 12?

 Link2
The Arg side chain consists of three nonpolar methylene groups and the strongly basic d-guanido group:
Link2
The Arg side chain consists of three nonpolar methylene groups and the strongly basic d-guanido group:
 With a p value usually of about 12, the guanido group is
ionized over the entire pH range in which proteins exist naturally. The
ionized guanido group is planar as a result of resonance:
With a p value usually of about 12, the guanido group is
ionized over the entire pH range in which proteins exist naturally. The
ionized guanido group is planar as a result of resonance:
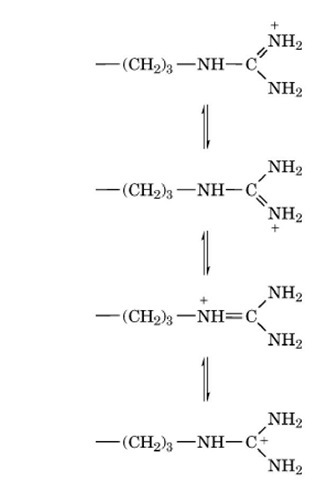
and the positive charge is effectively distributed over the entire
group. In the protonated form, the guanido group is unreactive, and only
very small fractions of the nonionized form are present at
physiological pH values. The guanido groups of Arg residues are almost
invariably at the surfaces of native protein structures, and virtually
no Arg residues are fully buried, but the nonpolar part of the side
chain, and the adjoining polypeptide backbone, are frequently buried
within the interior. Arg residues favor the alpha-helical conformation
in model peptides and also occur most frequently in that secondary
structure in folded protein structures.








