Hyperthermophilic Enzymes: Sources, Uses, and Molecular Mechanisms for Thermostability
Several properties of Arg residues suggest that they would be better adapted to high temperatures than Lys residues: the Arg δ-guanido moiety has a reduced chemical reactivity due to its high pKa and its resonance stabilization. The δ-guanido moiety provides more surface area for charged interactions than the Lys amino group does.Figure4 illustrates the ability of Arg to participate in multiple noncovalent interactions. Because the Arg side chain contains one fewer methylene group than Lys, it has the potential to develop less unfavorable contacts with the solvent.
Last, because its pKa (approximately 12) is 1 unit above that of Lys (11.1), Arg more easily maintains ion pairs and a net positive charge at elevated temperatures (pKa values drop as the temperature increases) (252, 354).
The average Arg/Lys ratios in the protein pools of the mesophiles and hyperthermophiles listed in Table Table4 (0.73 ± 0.37 and 0.87 ± 0.60, respectively) are associated with large standard deviations. (Among hyperthermophiles, Arg/Lys ratios vary from 0.52 in Aquifex aeolicus proteins to 2.19 in Aeropyrum pernix proteins.) These results suggest that if an increased Arg content is indeed stabilizing, this mechanism is not universally used among hyperthermophiles.
Stereo view of the ion pair between Arg19 and Asp111 in S. solfataricus indole-3-glycerol phosphate synthase. The Arg19 guanidinium group also forms a cation-π interaction with the Tyr93 π system and two H bonds with Thr84. Reprinted from reference 185 with permission of the publisher.
Arginine residues as stabilizing elements in proteins
Site-specific substitutions of arginine for lysine in the thermostable D-xylose isomerase (XI) from Actinoplanes missouriensis are shown to impart significant heat stability enhancement in the presence of sugar substrates most probably by interfering with nonenzymatic glycation. The same substitutions are also found to increase heat stability in the absence of any sugar derivatives, where a mechanism based on prevention of glycation can no longer be invoked. This rather conservative substitution is moreover shown to improve thermostability in two other structurally unrelated proteins, human copper, zinc-superoxide dismutase (CuZnSOD) and D-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) from Bacillus subtilis. The stabilizing effect of Lys----Arg substitutions is rationalized on the basis of a detailed analysis of the crystal structures of wild-type XI and of engineered variants with Lys----Arg substitution at four distinct locations, residues 253, 309, 319, and 323. Molecular model building analysis of the structures of wild-type and mutant CuZnSOD (K9R) and GAPDH (G281K and G281R) is used to explain the observed stability enhancement in these proteins. In addition to demonstrating that even thermostable proteins can lend themselves to further stability improvement, our findings provide direct evidence that arginine residues are important stabilizing elements in proteins. Moreover, the stabilizing role of electrostatic interactions, particularly between subunits in oligomeric proteins, is documented.
A structural role for arginine in proteins: multiple hydrogen bonds to backbone carbonyl oxygens.
We propose that arginine side chains often play a previously unappreciated general structural role in the maintenance of tertiary structure in proteins, wherein the positively charged guanidinium group forms multiple hydrogen bonds to backbone carbonyl oxygens. Using as a criterion for a "structural" arginine one that forms 4 or more hydrogen bonds to 3 or more backbone carbonyl oxygens, we have used molecular graphics to locate arginines of interest in 4 proteins: Arg 180 in Thermus thermophilus manganese superoxide dismutase, Arg 254 in human carbonic anhydrase II, Arg 31 in Streptomyces rubiginosus xylose isomerase, and Arg 313 in Rhodospirillum rubrum ribulose-1,5-bisphosphate carboxylase/oxygenase. Arg 180 helps to mold the active site channel of superoxide dismutase, whereas in each of the other enzymes the structural arginine is buried in the "mantle" (i.e., inside, but near the surface) of the protein interior well removed from the active site, where it makes 5 hydrogen bonds to 4 backbone carbonyl oxygens. Using a more relaxed criterion of 3 or more hydrogen bonds to 2 or more backbone carbonyl oxygens, arginines that play a potentially important structural role were found in yeast enolase, Bacillus stearothermophilus glyceraldehyde-3-phosphate dehydrogenase, bacteriophage T4 and human lysozymes, Enteromorpha prolifera plastocyanin, HIV-1 protease, Trypanosoma brucei brucei and yeast triosephosphate isomerases, and Escherichia coli trp aporepressor (but not trp repressor or the trp repressor/operator complex)
ps.
moiety 一半, (两个组成部分中的一)部分
Link1
5. Draw the predominant form of arginine at pH 7.4 and 12. Important pK's for the functional groups in this amino acid are α-carboxyl=2, δ-guanido=12, α-amino=9. What is the ratio of conjugate base/acid for the δ-guanido group in arginine at pH 7.4 and 12?


Link2
The Arg side chain consists of three nonpolar methylene groups and the strongly basic d-guanido group:

With a p value usually of about 12, the guanido group is ionized over the entire pH range in which proteins exist naturally. The ionized guanido group is planar as a result of resonance:
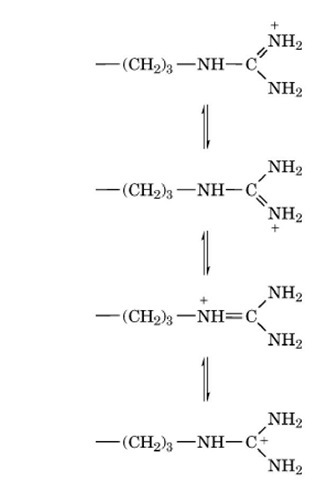
and the positive charge is effectively distributed over the entire group. In the protonated form, the guanido group is unreactive, and only very small fractions of the nonionized form are present at physiological pH values. The guanido groups of Arg residues are almost invariably at the surfaces of native protein structures, and virtually no Arg residues are fully buried, but the nonpolar part of the side chain, and the adjoining polypeptide backbone, are frequently buried within the interior. Arg residues favor the alpha-helical conformation in model peptides and also occur most frequently in that secondary structure in folded protein structures.

沒有留言:
張貼留言